Beyond Compliance:
The Pillars of GMP Certification
in Manufacturing Health Supplements

Good Manufacturing Practice (GMP) refers to a set of regulations and guidelines established by regulatory agencies, such as the Food and Drug Administration (FDA) in the United States. GMP compliance is essential to ensure the consistent production of safe and effective products following the public health guidelines.
Laboratoires Activa proudly adheres to these guidelines and holds GMP certification for the manufacturing of all its dietary supplements.
These guidelines define the minimum requirements for the manufacturing processes, facilities, equipment, personnel, and controls necessary to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, food, and other consumer products.
Key principles of GMP include:
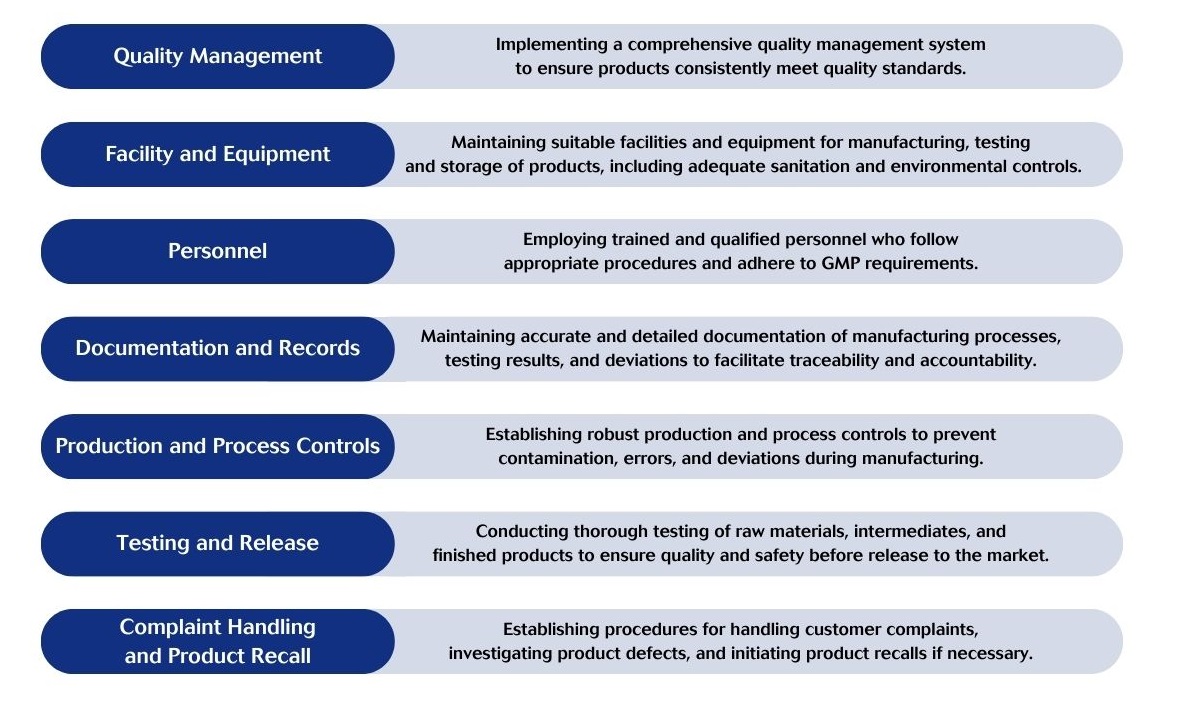
GMP certification is essential for ensuring the quality, safety, and efficacy of pharmaceuticals, medical devices, and other consumer products. This certification is renewed yearly following an inspection from the accredited delivery authorities.
By adhering to GMP guidelines, we demonstrate our commitment to maintaining rigorous standards throughout the production process. This certification provides assurance to worldwide regulatory agencies, healthcare professionals, and consumers that products are manufactured under controlled conditions and meet established quality criteria. Ultimately, GMP certification plays a crucial role in safeguarding public health and maintaining trust in the integrity of the manufacturing industry.