Joint support:
The synergy of active ingredients
for a comprehensive approach
Chondroitin and glucosamine are 2 compounds often used together in health supplements for joint support because they have complementary properties and benefits on joint health.
Glucosamine is naturally made by the body from amino acids, and is found in cartilage, and plays a key role in maintaining its integrity, structure and function in the joints.
Metabolic deficiency or ageing, may compromise the body’s cartilage production that becomes insufficient: degeneration sets in and leads the way to osteoarthritis. Glucosamine helps to stimulate the production of new cartilage and reduces the degradation of the existing one.
Several studies have shown that glucosamine hydrochloride reduces pain, improves mobility, and speeds healing in people with cartilage injuries[1,2]. It also helps to act on pain due to rheumatoid arthritis[3]. By increasing the lubrication of synovial fluids in the joints it helps slow cartilage degradation, unlike conventional anti-inflammatories which, in the long term, accelerate joint degeneration[4].
Chondroitin is naturally produced by the body and is an essential constituent of cartilage that provides hydration, elasticity, flexibility[5], and shock-absorbing properties to connective tissues. Several meta-analyses and syntheses concluded its effectiveness in relieving symptoms of mild to moderate osteoarthritis[6].
As a chondroprotective:
– It promotes the synthesis of cartilage cells and hyaluronic acid, allowing lubrication,
– It protects against enzymatic reactions and free radicals
– It could inhibit osteoclasts, responsible for bone loss.
Backed up by medical studies:
Studies have suggested that combining glucosamine and chondroitin may be more effective than taking either one alone. In 2005, a study published in the New England Journal of Medicine found that a combination of chondroitin and glucosamine was more effective at reducing pain and improving function in people with osteoarthritis than either supplement alone.
Two American studies conducted double-blind and controlled by placebo, indicating that the combined intake of glucosamine hydrochloride and chondroitin sulphate has been shown to be effective in relieving the symptoms of osteoarthritis of the knee[7,8].
The GAIT (Glucosamine/Chondroitin Arthritis Intervention Trial) specific study, conducted in 2010 for 24 weeks, obtained results in the same direction, on subjects suffering from moderate to severe pain[9].
Together, chondroitin and glucosamine work synergistically for joint support by helping to slow or stop the degeneration of joint tissues[10], and stimulate the synthesis of new cartilage, while regulating the enzymes responsible for their destruction.
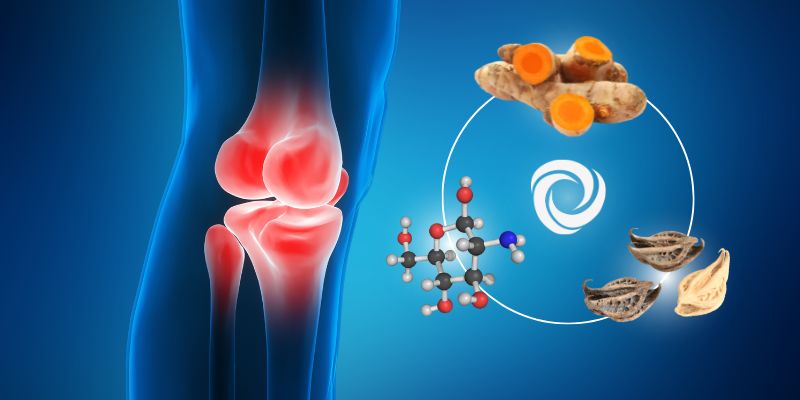
Glucosamine and Chondroitinhave a complementary action
to ‘TSE’ * on the protection of cartilage
Additionally, it has been found that combining glucosamine and chondroitin with other ingredients in a health supplement can further support joint health, help reduce inflammation and provide additional support for joint tissue.
Associating Turmeric Super Extract ‘TSE” (Curcumin extracted with a specific extraction technique to make it highly bioavailable to the body), with chondroitin and glucosamine can provide several benefits for joint health by potentiating the protective action of the cartilage provided by the TSE.
TSE has strong anti-inflammatory (as effective as NSAID), natural chondroprotective and antioxidant properties.
While each ingredient has its own unique properties, they work synergistically to provide joint support, including reducing inflammation, supporting cartilage health, improving joint mobility, and providing antioxidant protection. This comprehensive and synergistic approach results in greater overall benefits for joint health compared to using each ingredient individually.
Activa Joint Well-Being is the result of our research on the body’s ability to self-regulate in the face of dysfunction.
 In conclusion, Activa Well Being Joint, is the synergy of selected natural compounds to support a comprehensive and global approach to joint health.
In conclusion, Activa Well Being Joint, is the synergy of selected natural compounds to support a comprehensive and global approach to joint health.
Its purpose is to promote the protection and regeneration of cartilage as well as the reduction of inflammation and pain, and allows increased mobility. The formulation of Activa Well Being Joint is highly bioavailable and biocompatible.
Its galenic form, the extended release microgranule allows a reduced intake of 1 capsule to feel relieved for 8 hours.
If you are interested in this products
Sources:
[1] Braham R, Dawson B, Goodman C. The effect of glucosamine supplementation on people experiencing regular knee pain. Br J Sports Med.
[2] Glucosamine administration in athletes: effects on recovery of acute knee injury. Ostojic SM, Arsic M, et al. Res Sports Med. 2007 Apr-Jun;15(2):113-24.13.
[3] Nakamura H, Masuko K, et al. Effects of glucosamine administration on patients with rheumatoid arthritis. Rheumatol Int. 2007 Jan;27(3):213-8
[4] Towheed TE, Maxwell L, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005 Apr 18;(2):CD002946.
[5] J. Theodosakis. The Arthritis Cure
[6] Monfort J, Martel-Pelletier J, Pelletier JP. Chondroitin sulphate for symptomatic osteoarthritis: critical appraisal of meta-analyses. Curr Med Res Opin. 2008 May;24(5):1303-8.
[7] Das, A. and Hammad, T.A. Efficacy of a combination of FCHG49 glucosamine hydrochlo- ride, TRH122® low molecular weight sodium chondroitin sulphate and manganese ascorbate in the management of knee osteoarthritis. Osteoarthritis Cartilage. 2000 Sep;8(5):343-50.
[8] Leffler CT, Philippi AF, et al. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Mil Med. 1999 Feb;164(2):85-91
[9] legg DO, Reda DJ, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis, N Engl J Med, 2006 Feb 23;354(8):795-808.
[10] Clegg DO et coll. Glucosamine, chondroitin sulfate, and the two in combination for pain- ful knee osteoarthritis, N Engl J Med, 354(8) : 795-808